Ancora Heart Inc. Reaches First Enrollment Milestone in CORCINCH-HF Heart Failure Pivotal Trial
Ancora Heart Inc. Reaches First Enrollment Milestone in CORCINCH-HF Heart Failure Pivotal Trial
Six-month safety and effectiveness data for the 250 randomized patients to support PMA submission to FDA; enrollment to continue to 400
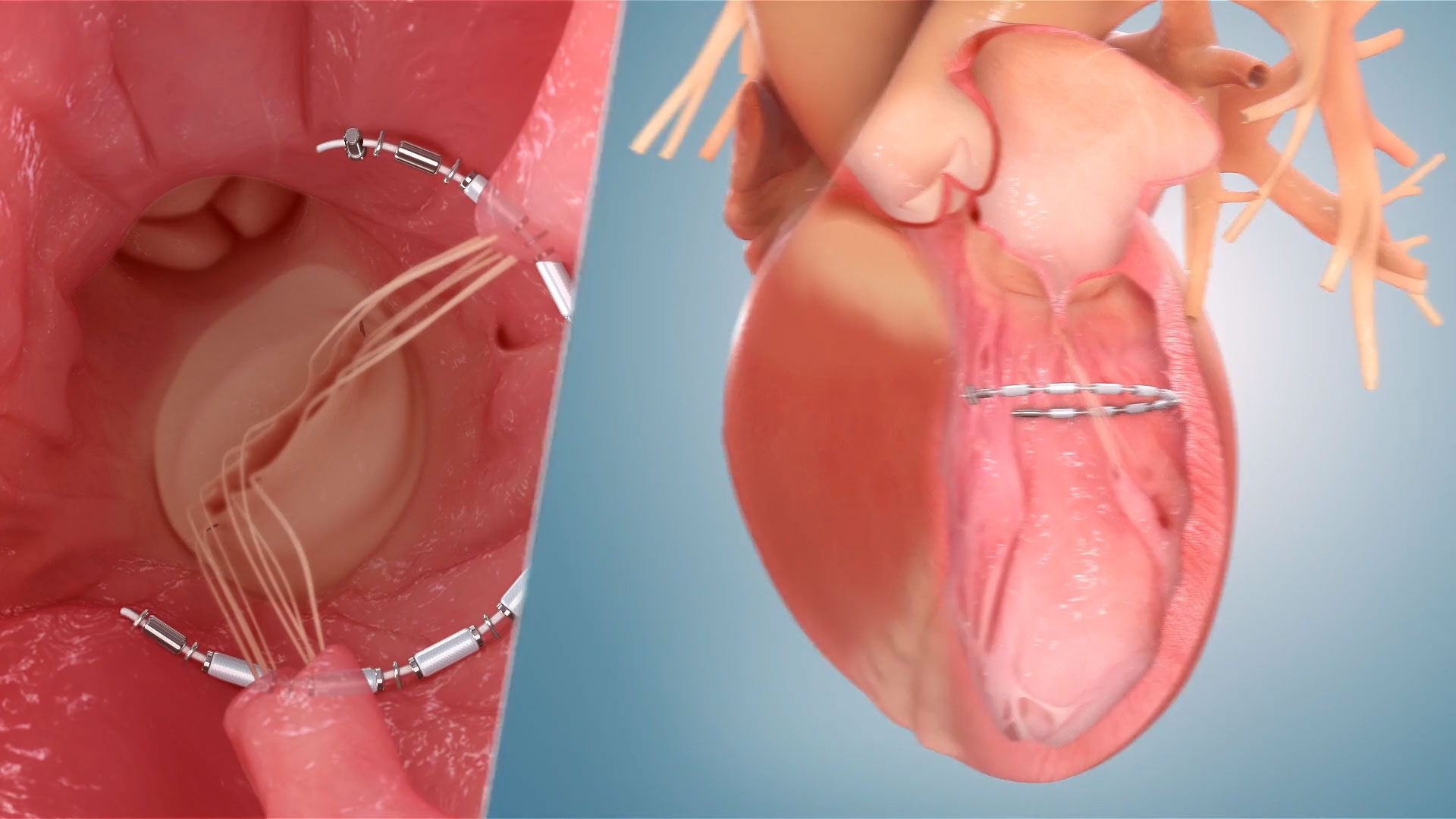
The AccuCinch® Transcatheter Left Ventricular Restoration System is an investigational device designed to reduce the size of the left ventricle, reduce ventricular wall stress, and support and strengthen the heart wall. The AccuCinch System is being studied in patients with heart failure with reduced ejection fraction (HFrEF) in the CORCINCH-HF pivotal trial.
SANTA CLARA, Calif.--(BUSINESS WIRE)--Ancora Heart, Inc., a medical device company developing a transcatheter device-based therapy to address heart failure (HF), today announced that it has reached the first enrollment milestone in the CORCINCH-HF pivotal trial evaluating the AccuCinch® Transcatheter Left Ventricular Restoration System in patients with heart failure with reduced ejection fraction (HFrEF). Six-month follow-up data on these 250 patients will support the company’s Premarket Approval (PMA) submission to the U.S. Food and Drug Administration (FDA).
“The AccuCinch System is the only completely transcatheter procedure to treat the enlarged left ventricle. Reaching this milestone is an incredible accomplishment in heart failure research." - Jeff Closs, president and CEO, Ancora Heart
Share
The AccuCinch System is an investigational device designed to reduce the size of the left ventricle, reduce ventricular wall stress, and support and strengthen the heart wall. Results from early clinical studies were presented at the 2023 Technology and Heart Failure Therapeutics conference and simultaneously published in the Journal of Cardiac Failure. The AccuCinch System was granted Breakthrough Device Designation from the FDA in 2022.
“The AccuCinch System is the only completely transcatheter procedure to treat the enlarged left ventricle,” said Jeff Closs, president and CEO of Ancora Heart. “Reaching this milestone is an incredible accomplishment in heart failure research and a model example of collaboration across heart failure and structural heart care teams at participating CORCINCH-HF clinical trial sites. We’d like to thank study investigators for their commitment to innovation and partnership, and we look forward to building on this momentum as we work toward reaching our full enrollment target of 400 patients.”
The CORCINCH-HF study is evaluating the safety and effectiveness of the AccuCinch System in patients who have symptomatic HF with reduced ejection fraction. An estimated 6.7 million adults in the U.S. live with heart failure, and about half have HFrEF.1,2
“Despite advances in guideline-directed medical therapy, many patients with heart failure continue to experience debilitating symptoms,” said Ulrich Jorde, MD, global co-principal investigator of the CORCINCH-HF Study, professor of medicine, Albert Einstein College of Medicine, and section head of Heart Failure, Cardiac Transplantation and Mechanical Circulatory Support at Montefiore Health System in New York. “Reaching this milestone in the CORCINCH-HF study is a significant step toward determining whether this treatment option may improve the length and quality of their lives.”
“AccuCinch is a device-based therapy aimed at reverse remodeling of the enlarged left ventricle,” said Mark Reisman, MD*, global co-principal investigator of the CORCINCH-HF Study, director of structural heart disease at NewYork-Presbyterian/Weill Cornell Medical Center and co-director of structural heart disease for NewYork-Presbyterian Queens and NewYork-Presbyterian Brooklyn Methodist Hospital, who was recruited to Weill Cornell Medicine as a professor of medicine. “This trial is designed to evaluate the safety of the device and procedure and whether we can improve heart structure and function and thereby help patients feel better, avoid hospitalizations and live longer.”
About Heart Failure
An estimated 6.7 million adults in the U.S. live with heart failure, a condition in which the heart’s muscles weaken and lose their ability to pump enough oxygen-rich blood to the body.1,2 Heart failure patients suffer from debilitating symptoms, including persistent exhaustion, trouble breathing and confusion, as well as frequent hospitalizations. About half of HF patients have heart failure with a reduced ejection fraction (HFrEF) and an enlarged left ventricle, the main pumping chamber of the heart, which causes more stress on the heart and leads to reduced pumping efficiency. Up to 50% of people who develop heart failure die within five years of diagnosis.3
About the AccuCinch® Transcatheter Left Ventricular Restoration System
The AccuCinch System is an investigational device designed to augment the existing care cardiologists provide their heart failure patients. For patients in whom HF has progressed beyond the ability of medications and pacemakers to manage symptoms, the AccuCinch System may provide a new treatment option to fill the gap between medication or pacemaker therapy and left ventricular assist devices (LVADs) or a heart transplant. During the minimally invasive AccuCinch System procedure, a flexible implant is attached to the inner wall of the left ventricle and then cinched. The implant is intended to reduce the size of the left ventricle, reduce ventricular wall stress, and support and strengthen the heart wall. The AccuCinch System is under clinical evaluation to determine its potential benefits related to patient symptoms, quality of life and life expectancy.
About the CORCINCH-HF Study
The CORCINCH-HF Study (NCT04331769) is a prospective, randomized, open-label, multicenter, international, clinical safety and effectiveness investigation of the AccuCinch Transcatheter Left Ventricular Restoration System, which is designed to enroll 400 patients at centers worldwide. The study has a unique design allowing initial analysis of safety and clinical effectiveness for PMA submission after the first 250 patients have reached six months of follow-up, and then a second analysis after the entire cohort has reached 12 months of follow-up.
About Ancora Heart, Inc.
Ancora Heart is a medical device company dedicated to providing new treatment options for people with heart failure (HF). The company’s lead product is the AccuCinch® Transcatheter Left Ventricular Restoration System, an investigational device currently being studied in the CORCINCH-HF pivotal trial. Ancora Heart is a privately held company located in Santa Clara, Calif. For more information, about Ancora Heart and its products, visit www.ancoraheart.com and follow the company on Facebook, LinkedIn and X.
_________________________________________ |
* Dr. Reisman reports travel support from Ancora Heart.
1 Martin SS, Aday AW, Almarzooq ZI, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee; Stroke Statistics Subcommittee. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149:e347–913.
2 Murphy S, Ibrahim N, Januzzi J. Heart Failure with Reduced Ejection Fraction, A Review. JAMA. 2020;324(5):488-504
3 Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-596.
Contacts
Media Contact:
Joni Ramirez, 323-532-0746
joni@merrymancommunications.com
