Triastek's 3D-Printed Gastric Retention Product T20G Receives FDA IND Clearance
Triastek's 3D-Printed Gastric Retention Product T20G Receives FDA IND Clearance
NANJING, China--(BUSINESS WIRE)--Today, Triastek announces that on February 27, 2025 a proprietary 3D-printed non-vitamin K antagonist oral anticoagulant (NOAC) product, T20G, received Investigational New Drug (IND) clearance from the U.S. Food and Drug Administration (FDA). This regulatory milestone follows the prior IND clearance of this product by China's National Medical Products Administration (NMPA) in January 2024.
Atrial fibrillation (AF), the most common type of treated heart arrhythmia prevalent in clinical practice, affects approximately 1%–2% of the general population, with global prevalence estimates ranging between 30 to 100 million cases. Anticoagulation therapy remains the cornerstone therapeutic intervention for stroke prevention in patients with AF. Among available therapeutic options, NOACs have emerged as the preferred pharmacologic agents due to their superior safety and efficacy profiles compared to traditional therapies. Reflecting this clinical advantage, NOACs are recommended as first-line treatment in leading guidelines, including those from the American Heart Association (AHA), European Society of Cardiology (ESC), and Asia-Pacific Heart Rhythm Society (APHRS).
T20G is being developed by using a novel dosage form (or drug delivery system) under the FDA's 505(b)(2) New Drug Application (NDA) pathway. Triastek holds exclusive global intellectual and commercialization rights for this innovative therapeutic candidate.
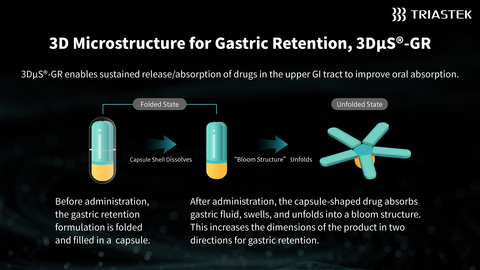
Leveraging Triastek's proprietary Melt Extrusion Deposition with Micro-Injection Molding (MED&MIM) process, T20G features the company's patented 3D Microstructure for Gastric Retention (3DμS®-GR) platform to enable once-daily oral administration. This optimized dosing regimen offers advantages over the twice-daily dosing required by the reference listed drug (RLD), potentially improving patient adherence and simplifying dosing management. During its gastric retention phase, T20G enables sustained release of the active pharmaceutical ingredient (API), facilitating drug absorption in the upper gastrointestinal tract, which significantly enhances its oral absorption.
Dr. Feihuang Deng, VP Technology of Triastek, said: “With T20G receiving IND clearance in both China and the U.S., Triastek has achieved a new milestone in the field of gastric retention drug delivery. Building on this dual regulatory recognition, we will accelerate the development of T20G to provide high-quality pharmaceutical products to patients worldwide.”
Contacts
Jiaxin Tao
Technical Marketing Specialist
taojiaxin@triastek.com