OMNIVISION Partners with Biotech Dental to Develop Highly Accurate 3D Intraoral Scanners
OMNIVISION Partners with Biotech Dental to Develop Highly Accurate 3D Intraoral Scanners
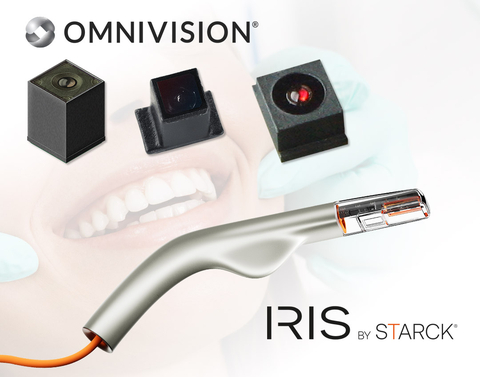
Together, the companies will improve dental visualization and patient care with Biotech’s Scan4All intraoral scanners that feature OMNIVISION’s miniature camera modules
SANTA CLARA, Calif. & SALON-DE-PROVENCE, France--(BUSINESS WIRE)--OMNIVISION, a leading global developer of semiconductor technology, including advanced digital imaging, analog, and display solutions, and Biotech Dental, a specialist in the design and manufacture of medical devices and digital solutions for dental applications, today announced a partnership that involves Biotech Dental’s use of multiple medical grade camera modules from OMNIVISION in the design of its new Scan4All 3D intraoral scanners Iris by Starck. The camera modules for dental applications feature OMNIVISION’s CameraCubeChip® technology with an integrated lens and will be showcased at the International Dental Show (IDS), March 25-29, 2025, taking place in Cologne, Germany (Hall 4.2/Booth #H038).
The Scan4All intraoral scanner Iris by Starck is a handheld device that is slightly larger than the size of an electric toothbrush; it uses sophisticated software to develop a 3D digital model of a patient’s teeth and gums, which can then be used to create dental restorations and implants. This method is significantly more convenient and accurate compared to the traditional dental putty approach. However, most intraoral scanners available today have only one image sensor, inhibiting their speed and accuracy. Biotech Dental is addressing this pain point by partnering with OMNIVISION to develop intraoral scanners with multiple image sensors that produce quick and accurate high-quality 3D dental models.
“We partnered with OMNIVISION due to the wide range of imaging solutions they offer for dental applications. Most importantly, OMNIVISION’s camera modules are developed and tested specifically for the medical and dental markets and have a long lifetime, which in turn, ensures that our intraoral scanners retain their value and remain a high-quality solution for our customers,” said Philippe VERAN, CEO, Biotech Dental.
According to Ehsan Ayar, medical product marketing manager, OMNIVISION, “Biotech Dental offers innovative, high-quality solutions for the dental industry. We are excited to partner with them to enhance their next-generation intraoral scanners, which will use several of our medical-grade high-resolution image sensors, taking dental visualization to the next level. We are the only company that offers a wide range of medical-grade imagers, which go through extensive biocompatibility, sterilization and medical tests. This gives dental professionals peace of mind during procedures.”
3D intraoral scanners are quickly replacing the use of traditional dental putty impressions, which are time consuming, complex and can be inaccurate. Data Bridge Market Research expects the global dental intraoral scanners market to grow with a compound annual growth rate (CAGR) of 11.1% from 2023 to 2030 and is expected to reach USD 1,325.80 million by 2030 from USD 573.76 million in 2022.1
Biotech Dental will utilize the following OMNIVISION CameraCubeChip® camera modules:
- The OCH2B30 camera module utilizes a square format CMOS image sensor with a 1500 x 1500 resolution at 60 frames per second (fps) or 720p resolution at 120 fps
- The OCHSA10 camera module offers 800 x 800 resolution with a frame rate of 60 fps or 400 x 400 resolution at 90 fps, for blur-free images and video
- The OVM9724 ultra-compact front-facing camera module captures 720p high-definition video at 30 fps
Both the OCH2B30 and OCHSA10 utilize OMNIVISION’s PureCel®Plus-S stacked-die technology, which enables their compact size. The OVM9724 is built on OMNIVISION’s powerful OmniBSI™+ pixel architecture to enable high-quality color images and fast-frame video. CameraCubeChip® is a reflowable, all-in-one camera drop-in solution—there is no need for additional optical components, which significantly streamlines manufacturing. The combination of excellent image quality and small form factor make the camera modules ideal for integration into slim, compact devices, such as the tip of the 3D intraoral scanner.
OMNIVISION is ISO13485-certified; the camera modules are tested for electromagnetic interference (EMC) and electromagnetic compatibility (EMI) compliance and sterilization. They are also biocompatible and waterproof. Due to OMNIVISION’s rigorous production testing, they are guaranteed to be within the stated electrical, mechanical and optimal specifications and do not require tuning or calibration.
For more information or to schedule a meeting at IDS, contact OMNIVISION sales: www.ovt.com/contact-sales.
About OMNIVISION
OMNIVISION is a global, fabless semiconductor organization that develops advanced digital imaging, analog and display solutions for multiple applications and industries, including mobile phones, security and surveillance, automotive, computing, medical, machine vision and emerging applications. Its award-winning, innovative technologies enable a smoother human/machine interface in many of today’s commercial devices. Find out more at www.ovt.com.
1Source: Data Bridge Market Research: Global Dental Intraoral Scanners Market – Industry Trends and Forecast to 2030. Published July 2023. https://www.databridgemarketresearch.com/reports/global-dental-intraoral-scanners-market
CameraCubeChip®, PureCel®Plus-S, OmniBSI™, OMNIVISION® and the OMNIVISION logo are trademarks or registered trademarks of OMNIVISION. All other trademarks are the property of their respective owners.
Contacts
OMNIVISION Media Contact:
Sandy Fewkes
Kiterocket
+1 408.529.9685
sfewkes@kiterocket.com
OMNIVISION Company Contact:
DeAnn Liu
OMNIVISION
+1 408.916.2536
PR@ovt.com